Full Time Aseptic Processing
• Closed means zero-CFU is minimal expectation
• Air and room quality outside doesn’t matter
 EVIDENCE-BASED ZERO CFUs
EVIDENCE-BASED ZERO CFUs
No opinions. No beliefs. No dogma. No superstition. No tradition. Just hard-core reproducible evidence.
Quality recognizes that every entire cell production line (all manual and automated steps) must be protected from microbial contamination by full-time aseptic conditions (zero CFU) instead of part-time by semi-aseptic (low CFU). The Cytocentric X2 Platform does this now.
 ASEPTIC PRODUCTION “TUNNEL” IS FITTED AROUND ENTIRE PRODUCT FLOW FROM BEGINNING TO END
ASEPTIC PRODUCTION “TUNNEL” IS FITTED AROUND ENTIRE PRODUCT FLOW FROM BEGINNING TO END
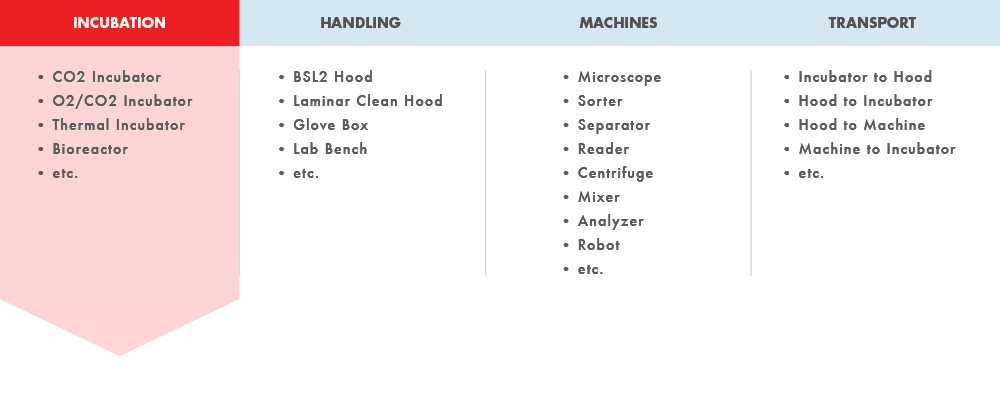
Metabolizing cells are found in only 4 places outside the body. Whether incubating, being processed or analyzed in machines, being handled, or being transported between any two, closed is the key to aseptic cell production.
CELLS OUTSIDE THE BODY
INCUBATION
• C02 Incubator
• 02/C02 Incubator
• Thermal Incubator
• Bioreactor
• etc.

HANDLING
• BSL2 Hood
• Laminar Clean Hood
• Glove Box
• Lab Bench
• etc.
MACHINES
• Microscope
• Sorter
• Separator
• Reader
• Centrifuge
• Mixer
• Analyzer
• Robot
• etc.
TRANSPORT
• Incubator to Hood
• Hood to Incubator
• Hood to Machine
• Machine to Incubator
• etc.
 LOW RISK INCUBATION
LOW RISK INCUBATION
Incubators open only into closed aseptic processing chamber with no people and no room air in the same space as cells. Technician can cough, sneeze, even spray sputum aerosols toward cells with no risk of contamination.
 HIGH RISK INCUBATION
HIGH RISK INCUBATION
Incubators open into room with people and HVAC air in the same space as cells. Minimally acceptable cGMP.
CELLS OUTSIDE THE BODY
INCUBATION
• C02 Incubator
• 02/C02 Incubator
• Thermal Incubator
• Bioreactor
• etc.
HANDLING
• BSL2 Hood
• Laminar Clean Hood
• Glove Box
• Lab Bench
• etc.

MACHINES
• Microscope
• Sorter
• Separator
• Reader
• Centrifuge
• Mixer
• Analyzer
• Robot
• etc.
TRANSPORT
• Incubator to Hood
• Hood to Incubator
• Hood to Machine
• Machine to Incubator
• etc.
 LOW RISK HANDLING
LOW RISK HANDLING
Closed with no people and no room air in the same space as cells. Technician can cough, sneeze, even spray sputum aerosols toward cells with no risk of contamination, yet not widely appreciated.
 HIGH RISK HANDLING
HIGH RISK HANDLING
Open with people and room air in the same space as cells, yet minimally acceptable.
CELLS OUTSIDE THE BODY
INCUBATION
• C02 Incubator
• 02/C02 Incubator
• Thermal Incubator
• Bioreactor
• etc.
HANDLING
• BSL2 Hood
• Laminar Clean Hood
• Glove Box
• Lab Bench
• etc.
MACHINES
• Microscope
• Sorter
• Separator
• Reader
• Centrifuge
• Mixer
• Analyzer
• Robot
• etc.

TRANSPORT
• Incubator to Hood
• Hood to Incubator
• Hood to Machine
• Machine to Incubator
• etc.
 LOW RISK MACHINE PROCESSING
LOW RISK MACHINE PROCESSING
Closed with no people and no room air in the same space as cell processing machine and cells. With no effort, machine stays clean and cells never get contaminated, yet not widely appreciated.
 HIGH RISK MACHINE PROCESSING
HIGH RISK MACHINE PROCESSING
Open with people and room air in the same space as cells and machine, yet minimally acceptable.
CELLS OUTSIDE THE BODY
INCUBATION
• C02 Incubator
• 02/C02 Incubator
• Thermal Incubator
• Bioreactor
• etc.
HANDLING
• BSL2 Hood
• Laminar Clean Hood
• Glove Box
• Lab Bench
• etc.
MACHINES
• Microscope
• Sorter
• Separator
• Reader
• Centrifuge
• Mixer
• Analyzer
• Robot
• etc.
TRANSPORT
• Incubator to Hood
• Hood to Incubator
• Hood to Machine
• Machine to Incubator
• etc.

 HIGH RISK CELL TRANSPORT
HIGH RISK CELL TRANSPORT
Open with people and room air in the same space as cells, yet minimally acceptable.
Quality recognizes that zero CFU must be easily maintained regardless of air quality of the room, including unclassified clinical settings. The Xvivo GMP System® Platform does this now.
EVIDENCE: Regardless of Conditions Outside Xvivo System, Inside is always ISO-5/Grade A or better.

Quality recognizes that zero CFU must be reliably maintained by low-skill production technicians. The Cytocentric X2 Platform does this now.
COMPARATIVE RISK ASSESSMENT: Regulatory agencies understand it. Live cell industry doesn’t.
| CONTAMINATION RISK | XVIVO GMP+ | OPEN cGMP |
|---|---|---|
| Room Air | ||
| Technicians | ||
| Surface | ||
| Sample |
PRETTY LITTLE SECRET: One risk – surfaces of materials going in.
| CONTAMINATION RISK | XVIVO GMP+ | OPEN cGMP |
|---|---|---|
| Room Air | ||
| Technicians | ||
| Surface | ||
SURFACE CONTAMINATION RISK MITIGATION
MULTIPLE REDUNDANT CONTROL POINTS
1. DECON WASH HANDS & GLOVE UP (decon wipe gloves).
2. STAGE MATERIAL NEAR LAMINAR HOOD
3. PREP LAMINAR HOOD (decon wipe floor of hood and gloves)
4. RE-STAGE MATERIAL INTO FRONT OF HOOD
5. MATERIAL PREP & RE-STAGE (TRANSFER) INTO REAR OF LAMINAR (debag, decon wipe all surfaces of all materials)
6. PREP BUFFER CHAMBER (decon wipe knob and floor)
7. MATERIAL TRANSFER INTO BUFFER AND CLOSE
8. WAIT FOR DF (detached microbes will be cleared in 10 mins)
9. PREP PROCESSING CHAMBER (decon wipe fixed gloves/overgloves, floor, buffer knob)
10. MATERIAL TRANSFER INTO PROCESS CHAMBER (decon wipe bottom surface of materials)
11. WAIT CRAC AND/OR DF (detached microbes will be cleared in 10 min)
12. PROCESS CULTURES
13. WAIT CRAC (detached microbes and aerosols will be cleared in 10 min)
14. TRANSFER CULTURES INTO INCUBATOR (decon wipe incubator door knob and front edge of sliding tray)
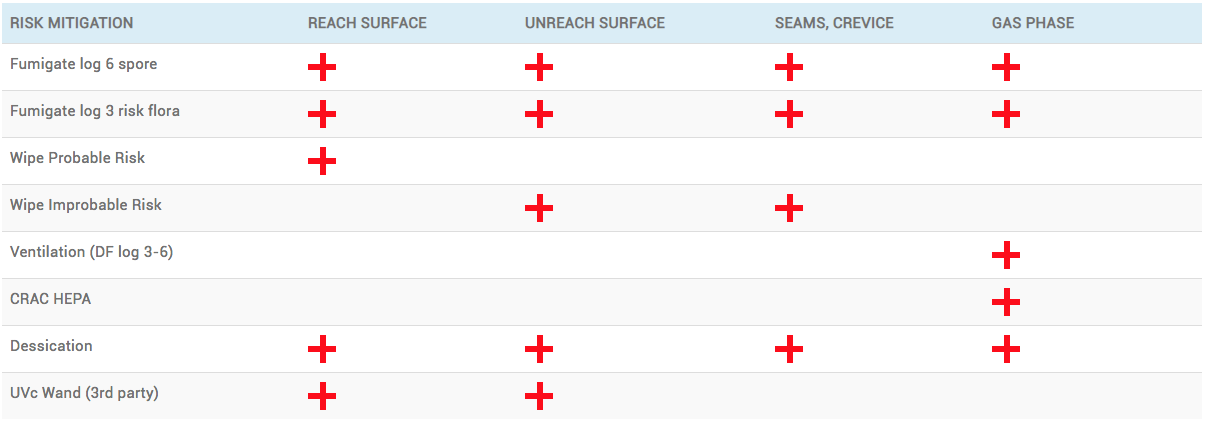
PROCESS CHAMBERS: MULTIPLE REDUNDANT CONTROL POINTS
| RISK MITIGATION | REACH SURFACE | UNREACH SURFACE | SEAMS, CREVICE | GAS PHASE |
|---|---|---|---|---|
| Fumigate log 6 spore | ||||
| Fumigate log 3 risk flora | ||||
| Wipe Probable Risk | ||||
| Wipe Improbable Risk | ||||
| Ventilation (DF log 3-6) | ||||
| CRAC HEPA | ||||
| Dessication | ||||
| UVc Wand (3rd party) |
INCUBATION CHAMBERS: MULTIPLE REDUNDANT CONTROL POINTS
| RISK MITIGATION | REACH SURFACE | UNREACH SURFACE | SEAMS, CREVICE | GAS PHASE |
|---|---|---|---|---|
| Fumigate log 6 spore | ||||
| Fumigate log 3 risk flora | ||||
| Wipe Probable Risk | ||||
| Wipe Improbable Risk | ||||
| Ventilation (DF log 3-6) | ||||
| Microbicidal water bath | ||||
| Microbicidal interior | ||||
| Autoclave rack, shelves |
BUFFER CHAMBERS: MULTIPLE REDUNDANT CONTROL POINTS
| RISK MITIGATION | REACH SURFACE | UNREACH SURFACE | SEAMS, CREVICE | GAS PHASE |
|---|---|---|---|---|
| Fumigate log 6 spore | ||||
| Fumigate log 3 risk flora | ||||
| Wipe Probable Risk | ||||
| Wipe Improbable Risk | ||||
| Ventilation (DF log 3-6) | ||||
| Dessication | ||||
| UVc Wand (3rd party) |
 LOW RISK CELL THERAPY MANUFACTURING
LOW RISK CELL THERAPY MANUFACTURING
No people and no room air are ever in the same space as clinical grade cells.
 HIGH RISK CELL THERAPY MANUFACTURING
HIGH RISK CELL THERAPY MANUFACTURING
People and room air are always in the same space as clinical grade cells. It doesn’t make sense, but people-centric never does when it comes to cells. It’s minimally acceptable, until something better like Cytocentric comes along.